http://www.oxygen-products.com/images/MazzeiOzoneDisinfectionWater2008.pdf
Ozone Disinfection And Public Health: A Historical Prospective
To accurately predict the role ozone will play in the future of disinfection, it is important to first
understand its historical impact on drinking water treatment over the past 100 years.
BY JAMES R. JACKSON
Few environmentalists would categorize ozone an “environmental friendly” technology. A byproduct of smog irradiation by sunlight, ozone is used by the U.S. Environmental Protection Agency (EPA) as an indicator of ground-level air pollution. However, the EPA also endorses the use of ozone gas as a disinfectant in municipal drinking water plants.
Ozone’s climb from pollutant to drinking water disinfectant was not quickly or easily achieved, particularly in the United States. It took decades of successful use in Western Europe, as well as a U.S. epidemic, before ozone was finally embraced as a standard disinfection method in the United
States. This article provides a brief account of ozone’s historic role in drinking water disinfection and looks ahead to the role it will play in protecting public health in the future.
________________________________________ |
Western Europe Pioneers Ozone Disinfection
German chemist Christian Friedrich Schönbein first generated ozone by passing dry air across an electrode in 1840. Schönbein discovered that oxygen molecules cleaved by an electric spark recombined to form a pungent, pale blue gas composed of three oxygen atoms, which he called ozone (from the Greek word ozein, which means “to smell”).
Later experiments showed that when ozone was dissolved in water, it rapidly killed microorganisms and then reverted back to its parent molecule, oxygen. This discovery led to the first use of ozone in a drinking water plant, when the city of Nice, France, began using it to disinfect the public water supply in 1906. As a result of the Nice plant’s success, ozone became the primary drinking water disinfectant throughout Western Europe during the early 20th century.
________________________________________ |
United States Slow To Adopt Ozone
While ozone was on the rise in Europe, chlorine was establishing itself as the disinfectant of choice for public drinking water treatment in the United States. At the same time Nice was setting up its historic ozone plant, the Jersey City, NJ, water works was looking for a reliable method for disinfecting public drinking water to reduce the incidence of waterborne illness.
The Jersey City government was aware of the ozone installation in Nice, but decided the use of ozone was too complex and costly. Consequently, it selected sodium hypochlorite, a low-cost liquid derivative of chlorine gas, as its primary disinfectant in 1908. The successful eradication of
waterborne pathogens by chlorine treatment led to federal regulations requiring U.S. public drinking water systems to maintain a chlorine residual.
________________________________________ |
Milwaukee Tragedy Puts Ozone In The Spotlight
Chlorine remained the primary disinfectant used by U.S. drinking water plants until 1993, when contamination of the Milwaukee, WI, drinking water supply caused widespread gastrointestinal illness. The contaminated water afflicted more than 400,000 Milwaukee residents with stomach
cramps, diarrhea, and vomiting. By the time the outbreak subsided, it had claimed the lives of 111 people.
Investigators from the Centers for Disease Control and Prevention (CDC) quickly determined that a parasite shed in animal feces, Cryptosporidium parvum, was responsible for the epidemic. Additional research revealed that the pathogenic microorganism was highly resistant to chlorine.
The discovery of a waterborne, chlorine-resistant pathogen shocked regulators — the public water supply was no longer safe.
In response, the EPA began looking for another disinfectant that could eliminate Cryptosporidium from public drinking water systems. Based on its long history of success in Western European water plants, ozone was moved to the top of the list as a possible Cryptosporidium disinfectant. Extensive government-sponsored research eventually determined that a small amount of ozone would destroy Cryptosporidium in less than 10 minutes.
________________________________________ |
U.S. EPA Promulgates Ozone Disinfection
The effectiveness of ozone on Cryptosporidium led the EPA to make significant changes to U.S. drinking water regulations. These changes are reflected in the Long Term 2 Enhanced Surface Water Treatment Rule (LT2ESWTR), which was proposed by the EPA in July 2003 and finally
adopted as law in January 2006.
The LT2ESWTR requires all public water systems that use surface water, or ground water under the influence of surface water (aquifers containing surface water sources), to achieve a 99% to 99.9% (2- to 3-log) inactivation of Cryptosporidium. The term “inactivation” is used because when the 3 µm Cryptosporidium oocyst is stained with fluorescent dye and viewed under a dark field microscope, an infectious oocyst and an oocyst terminated through exposure to dissolved ozone
appear the same.
Consequently, the only way to confirm inactivity is to inoculate a laboratory animal with the recovered parasite and wait for symptoms of infection to appear. To make compliance easier for municipal water plants, the EPA has published tables that relate exposure of Cryptosporidium to a dissolved ozone concentration over a specific period of time, to the inactivation of the pathogen.
These CT tables, as they are known, specify that the product of dissolved ozone concentration (C) over time period (T) — measured in minutes — will statistically inactivate a certain percentage of the Cryptosporidium oocysts that may reside in the public water system. The percentage of oocysts inactivated are stated as log credits, in which the water plant claims
credit for statistically removing a given number of the oocysts by achieving a specific CT stated in units of concentration (mg/L) x minutes of contact time (minutes).
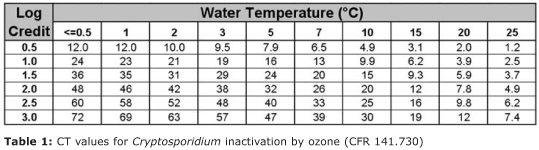
Table 1 shows the CT table currently used by municipal ozone installations. Because the speed of ozone disinfection varies with water temperature, the required CT (contained within the main body of the table) increases as the water temperature drops.
For example, to obtain a 2-log credit (99% oocyst inactivation) in 20°C water, the water plant must achieve a CT of 7.8 (dissolved ozone concentration x minutes of contact time). A water
temperature drop to 10°C, however, increases the CT requirement to 20. This increase in CT requirement with falling water temperatures is related to the speed at which molecular ozone will move from the water and into the oocyst cell: the colder the water, the slower the activity of ozone. Consequently, public water systems located in colder regions of the United States must construct extensive ozone contact basins to insure they achieve adequate CT values during winter operation.
________________________________________ |
Ozone’s Future
Drinking water dominated the news headlines in early 2008, when investigations revealed that trace levels of pharmaceuticals are present in U.S. drinking water supplies. To members of the water treatment industry, this news came as been dedicated to the issue of pharmaceutical agents in drinking water.
That discussion and research has heated up in recent months, however, as the general public has started paying more attention to the problem, and as municipal wastewater plants continue to discharge pharmaceutical agents into the primary water source for the majority of U.S. public drinking water systems — rivers and streams.
For example, pharmaceutical agents known as endocrine disruptors pose a serious threat to wildlife and human health. Passed through the U.S. population and into wastewater plants, endocrine disruptors are unaffected by standard wastewater treatment methods. Estrogen excreted in the urine of women using oral contraceptives, for instance, is suspected of causing the feminization of entire fish populations.
When exposed to a few parts per billion of contraceptive estrogen, male fish can actually become female. In a drinking water plant, the low-dosage ozone and chlorine treatments used in disinfection are unable to destroy endocrine disruptors, so they are passed through to the drinking water supply.
Medical research has shown that these pharmaceutical agents can disrupt the body’s endocrine system, affecting the development of young people and impacting the reproductive and neurological functions of adults. As a result, the EPA has made the removal of endocrine disruptors from drinking water a top priority.
Research into treatment methods for removing endocrine disruptors from wastewater effluent is ongoing, with ozone on the list of potential candidates. As research into the removal of endocrine disruptors — and other pharmaceutical agents — continues, ozone could emerge as a costeffective method for converting harmful agents in municipal wastewater effluent into harmless compounds, helping to ensure the safety of our public drinking water supply.
________________________________________ |
by James R. Jackson
James R. Jackson is the national manager of water and wastewater systems for Mazzei Injector Corporation. He has more than 25 years of water and wastewater treatment experience and has been working in the ozone industry as an ozone application specialist since 1994. He is also a licensed water and wastewater plant operator in Arizona and has been active in many trade associations, including the American Water Works Association Water Association (IBWA), and International Ozone Association (IOA). Jackson received his BS degree from Wayne State University.
http://www.oxygen-products.com/images/MazzeiOzoneDisinfectionWater2008.pdf
|